Biopharmaceutical product development demands rigorous adherence to regulatory frameworks encompassing Good Laboratory Practice (GLP), Good Manufacturing Practice (GMP), and Good Clinical Practice (GCP), collectively known as GxP. The FDA, EMA, and ICH set global standards for assay development and validation to ensure that analytical methods are robust, reproducible, and compliant.
Assay validation aligns with ICH Q2(R2) guidelines and includes assessment of parameters such as accuracy, precision, specificity, linearity, range, and robustness. These criteria guarantee the reliability of data used for critical decisions in drug development, regulatory submissions, and quality control throughout the product lifecycle.
Biomarkers provide essential insights into biological processes, disease mechanisms, and therapeutic efficacy. Their qualification requires sensitive, specific, and validated analytical methods. Technologies such as quantitative PCR (qPCR) enable precise nucleic acid quantification, protein arrays facilitate multiplexed detection of protein markers, and LC-MS panels provide in-depth proteomic profiling with high sensitivity and specificity.
Qualified biomarker assays are pivotal in translational research for monitoring pharmacodynamics, patient stratification, and safety assessments, thereby accelerating the development of personalized medicine and targeted therapies.
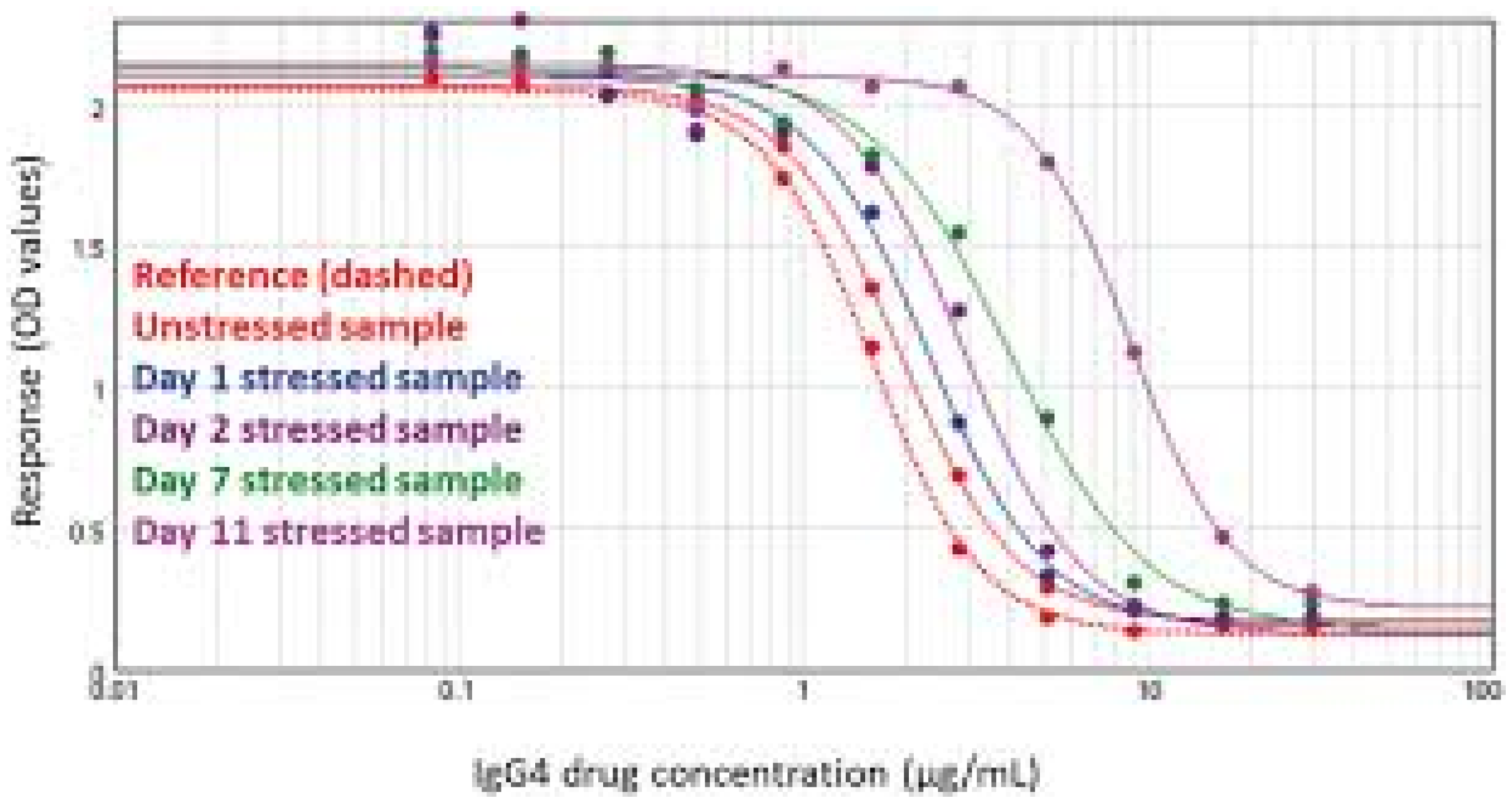
Potency assays are indispensable in confirming the biological activity of therapeutic proteins such as cytokines, monoclonal antibodies, and biosimilars. Regulatory agencies emphasize the use of bioactivity assays that reflect the mechanism of action relevant to clinical efficacy.
These assays commonly involve cell-based functional tests that measure responses such as receptor activation, cell proliferation, or immune effector functions (e.g., ADCC). The development and validation of these assays ensure reliable lot release, comparability between biosimilars and reference products, and ongoing monitoring of product stability and efficacy.